ServicesEnd-to-End CDMO Services based on technological Expertise
- GMP Manufacturing
-
KOLON Biotech has excellent capability in dealing with various types of ATMP products like cell and gene therapy and exosome therapy. we can satisfy any specific needs of our customers at every stage of biopharmaceutical development.We can address any manufacturing hurdles in every step of product life cycle.We’re able to conduct a new process adaptation by Tech Transfer and Scale out or Scale up, Future Area for Capacity buildup.
Therapy Type
-
-
1
Somatic cell
-
2
Stem cell(MSC, iPSC, etc.)
-
3
Exosome
-
1
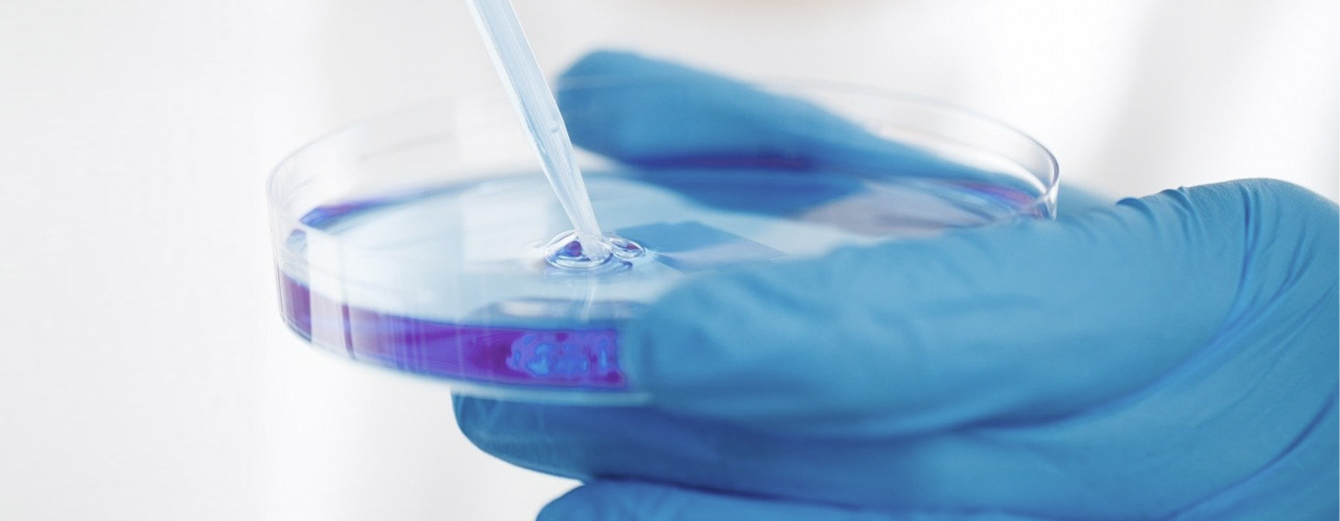
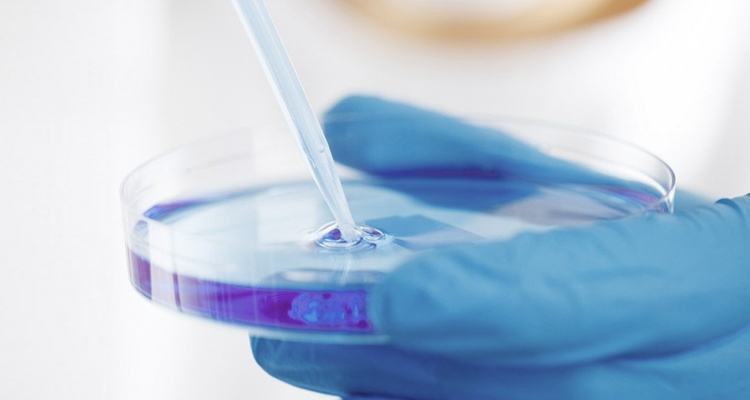
Track Record
- KOLON Biotech has accumulated a strong track record providing CDMO services to various customers for a wide range of biopharmaceutical products.
exampleSelective examples
| No. | Product |
|---|---|
| 1 | Cell Therapy |
| 2 | Gene-modified Cell Therapy |
| 3 | Gene-modified Stem Cell Therapy |
| 4 | Exosome Therapy |
| 5 | Stem Cell Therapy |
| No. | Product | Service | Phase | ||
|---|---|---|---|---|---|
| Process Development |
GMP Manufacturing |
Storage | |||
| 1 | Cell Therapy | check |
check |
check |
Clinical / Commercial |
| 2 | Gene-modified Cell Therapy | check |
check |
check |
Clinical / Commercial |
| 3 | Gene-modified Stem Cell Therapy | check |
check |
Clinical | |
| 4 | Exosome Therapy | check |
check |
check |
Clinical |
| 5 | Stem Cell Therapy | check |
check |
check |
Clinical |
Scale-up Technology
- 2D Cell Production Platform (Developing)
- manufacturing adherent cells in large scale with minimum cost and failure risk
-
 T-flask
T-flask -
 Medium Scale
Medium Scale
multi-layer flask -
 Large Scale Culture
Large Scale Culture
vessel w/ automated
device -
 Continuous
Continuous
Centrifuge -
 Bag-type
Bag-type
filtration -
 Vial filling
Vial filling
-
 T-flask
T-flask -
 Medium Scalemulti-layer flask
Medium Scalemulti-layer flask -
 Large Scale Culture vessel w/ automated device
Large Scale Culture vessel w/ automated device
-
 ContinuousCentrifuge
ContinuousCentrifuge -
 Bag-typefiltration
Bag-typefiltration -
 Vial filling
Vial filling
- Closed process addresses the risk of contamination during the open process
- Semi-automated manipulation ensures high batch-to-batch consistency & reduced processing time
- 3D Cell Production Platform (Developing)
- Conversion of adherent cell culture method from 2D to 3D using microcarrier & bioreactor